Achieving your target hemoglobin A1C when you have diabetes can be difficult, and even more so if you have a fear of hypoglycemia (low blood glucose), as many do. The natural reaction is to allow blood sugar levels to remain high in order to prevent low glucose episodes. However, in the long run, we know this is more harmful than helpful. The CGMS, or continuous glucose monitoring system, can help. This tool has been around now for years. But we learn more and more about the benefit of this important tool as more research is done and as the systems themselves advance.
There are two different types of continuous glucose monitors (CGMs). The “professional” CGMs and the “personal” CGMs. Professional CGMs are provided by your healthcare provider and worn temporarily for a fixed period of time, usually about 5 days. The user is blind to the data. Data is then downloaded and analyzed by the healthcare provider and used to make medication or diet adjustments. We offer this tool here at Texas Diabetes and Endocrinology and find it to be invaluable in helping our patients safely improve control over their diabetes.
Personal CGMs are purchased by the user and worn at the user’s discretion, up to 7 days at a time. These sensors provide real time data so that adjustments can be made immediately. The user is notified by an alarm if the glucose decreases below or rises above a certain threshold, prompting a glucose finger stick and immediate treatment depending on the glucose level.
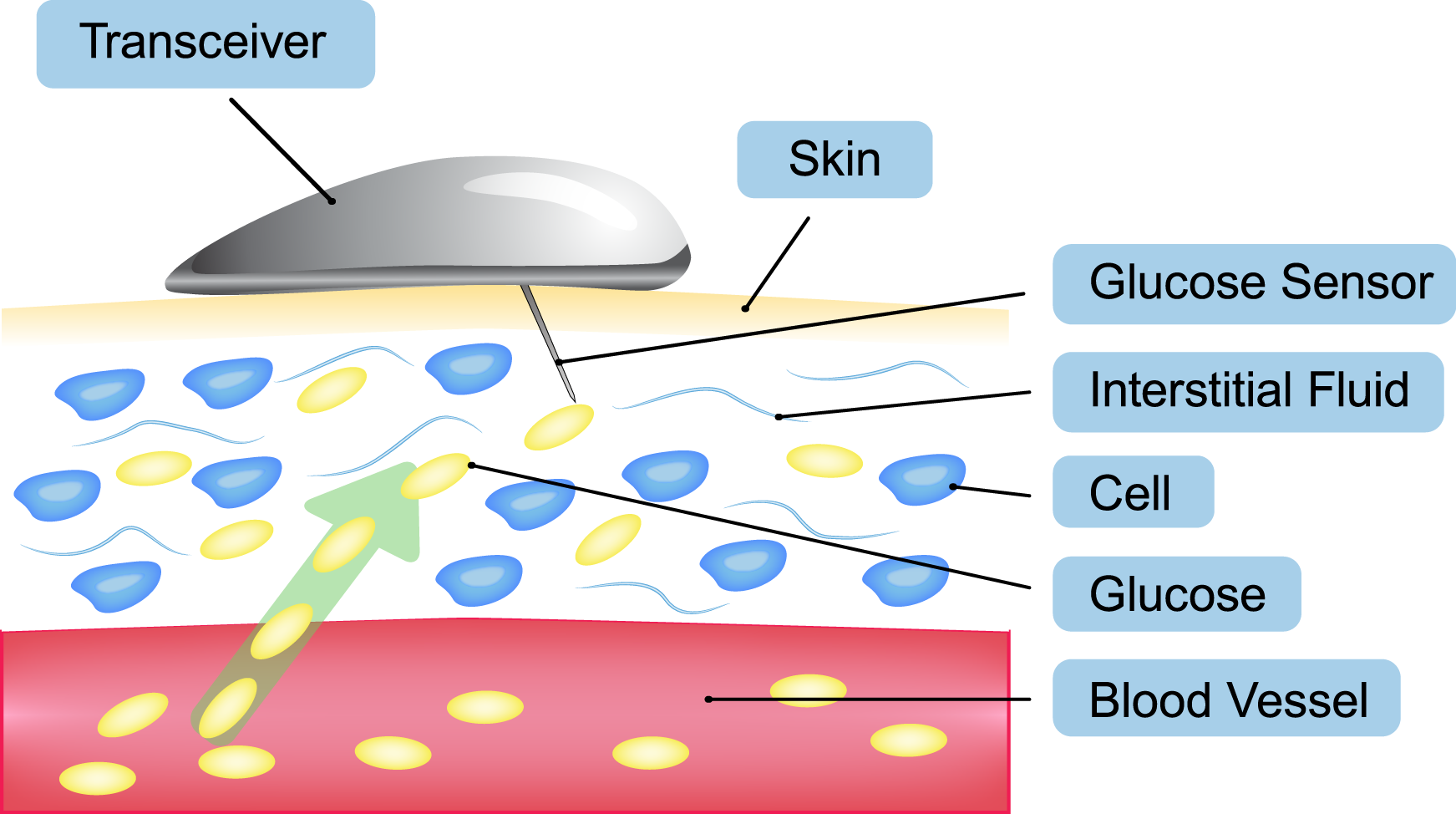
CGM systems use a very thin metallic filament (the sensor) that is inserted just below the skin to detect glucose in the subcutaneous interstitial fluid. The information from the sensor is then transmitted to a handheld receiver (smaller than most cell phones) which displays the glucose level and whether the glucose level is trending up or down.
The American Association of Clinical Endocrinologists recommends personal CGM for the following adult patients (guidelines issued in 2010):
Those with Type 1 DM and the following:
-hypoglycemia unawareness or frequent hypoglycemia judged to be excessive, potentially disabling, or life-threatening
-Excessive glycemic variability
-Requiring HbA1C reduction without increased hypoglycemia
-during pre-conception and pregnancy
Several research trials have shown improvement in A1C in those using a CGMS in addition to self-monitoring of their glucose compared to those self-monitoring their glucose alone.