News
FDA approves first automated insulin-delivery device for type 1 diabetes
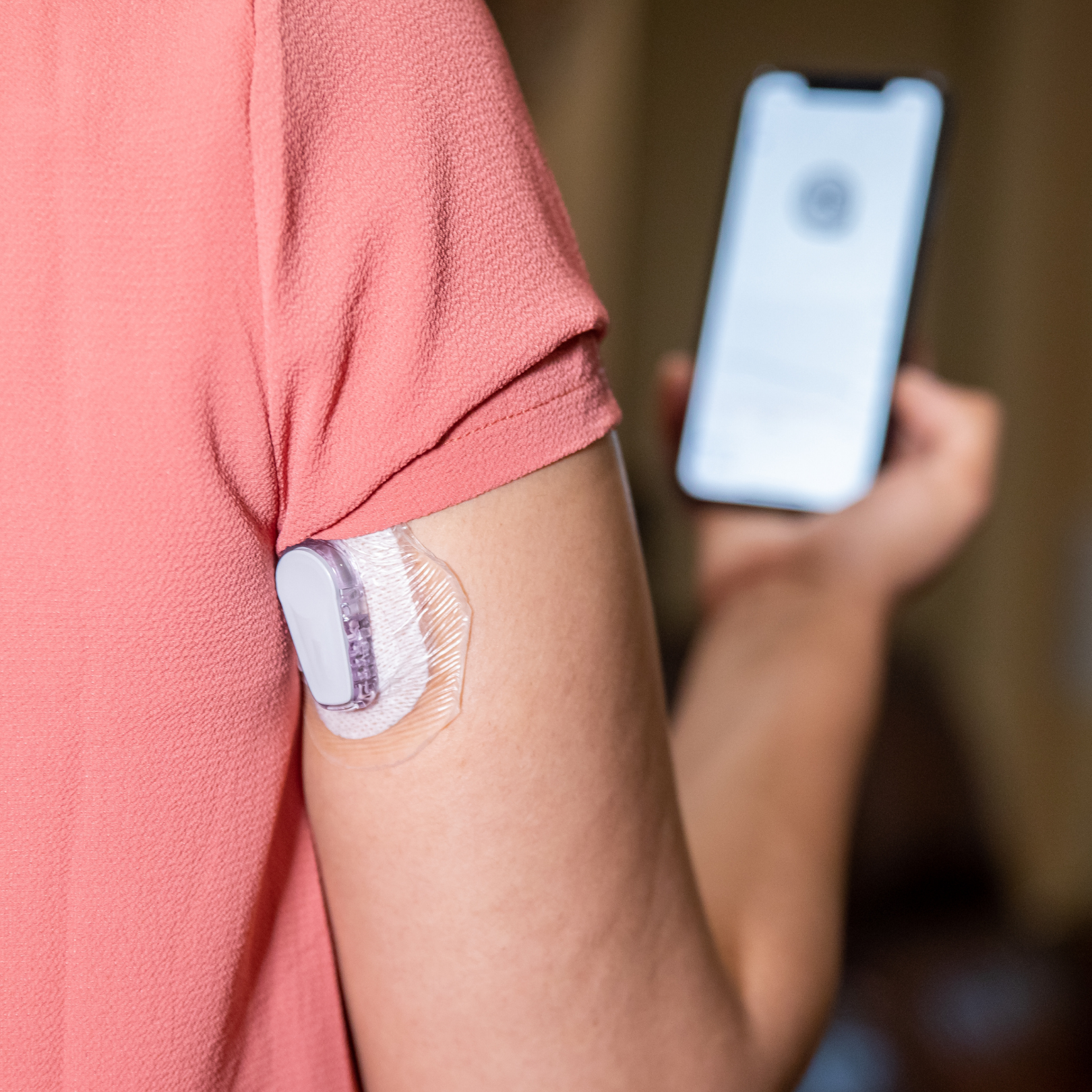
The FDA announced the approval of Medtronic’s MiniMed 670G, the first hybrid closed loop system – often referred to as an “artificial pancreas” – on September 28th. The device is designed to check sugar levels, and provide appropriate insulin doses, based on those readings. It is currently approved for use for people aged 14 years and older with type 1 diabetes.
The FDA emphasized its dedication to making technologies available that can help improve the quality of life for those with chronic diseases — especially those that require day-to-day maintenance and ongoing attention in a press release. The press release said: “This first-of-its-kind technology can provide people with type 1 diabetes greater freedom to live their lives without having to consistently and manually monitor baseline glucose levels and administer insulin.”
The new device measures glucose every five minutes and automatically administers or withholds insulin, based on these levels. Users will still need to manually administer insulin doses for mealtime insulin.
The system includes a sensor that monitors glucose levels under the skin, an insulin pump, and an infusion patch.
Data from a clinical study including 123 participants with type 1 diabetes showed safety and effectiveness of the device for people aged 14 years and older.
An ongoing study is currently looking at the safety and effectiveness of the system for children aged 7 to 13 years with type 1 diabetes.
More information about the device can be found here: http://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=2206594